The octet rule is a useful guideline when drawing Lewis structures. For a molecule uncharged that count is the correct.
Lewis Electron Dot Structures Help
First of all a correct count of all valence electrons is essential.

. Rules for drawing Lewis structures. Instructions for Drawing Lewis Structures For molecules and polyatomic ions composed of nonmetals. Count the total number of valence electrons.
Most often you will see H C O N as central atoms so I have come up with something I call the HONC Rule shown below. 11 hours agoDraw the correct Lewis Structures for each molecule on the list below and name each molecule following the rules for naming. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots.
Drawing Lewis Structures A step-by-step Guide by C. Combining atoms to form covalent molecules can be accomplished following five easy steps. You can print and fill this table to complete the answers for this question or you can copy the table in a piece of paper and fill in your answers.
Less than an octet of valence electrons. Rules for Drawing Lewis Structures For molecules known to exist follow these rules to determine the Lewis structure. Find the Total Number of Valence Electrons.
Draw the initial skeletal structure with atoms connected by single bonds. General rules for writing LEWIS structures for molecules. -Hydrogen cannot be central.
Rules for drawing Lewis dot structures. -Atoms that occur once or first are usually central. Rules for Drawing Lewis Structure.
Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure. Determine the total number of valence electrons in the molecule or ion. Drop the -ne ending of the alkane to get the root name hexa-.
It should be the most symmetric structure you can draw. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
Determine the Number of Bonds in the Molecule. Count the number of valence e- each atom brings into the molecule. Put electron pairs about each atom such that there are 8 electrons around each atom octet rule.
Try the following as a first guess. Determine the central atom and write the skeleton structure of the molecule. Count the total number of valence electrons in the structure Remember Group of Valence electrons 2.
A major resonance contributor is one that has the lowest energy. The most stable structures contribute most to the resonance. For ions the charge must be taken into account.
O S Se Te Be. CBr 4-The least electronegative atom is central. The SUM TOTAL is what is important.
Sometimes the number of valence e- is odd. Rules for drawing Lewis structures. Find the Number of Electrons Needed to Make the Atoms Happy Step 3.
All valence electrons of the atoms in Lewis structures must be shown. We can often write more than one Lewis structure for a molecule differing only in the positions of the electrons. N P As Sb.
Draw a Skeletal Structure. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. The following rules are given to assist you.
First of all a correct count of all valence electrons is essential. H F Cl Br I. When the halogens are terminal atoms they form single bonds.
For a molecule uncharged that count is the correct. If ionic treat each ion separately. C N O S Cl Br and I.
Learn vocabulary terms and more with flashcards games and other study tools. Group number for each element of valence electrons. Solved when you draw the Lewis Structure for CH3OCH2CH3 Chegg.
The least electronegative atom will be central. Add electrons for negatively charged ions. What are the 6 rules for drawing Lewis dot structures.
MUST FOLLOW IN ORDER. Hoeger 1 Determine the total number of valence electrons for ALL atoms. Odd number of electrons.
Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds. 1 Life and the Chemistry of Carbon CompoundsWe Are Stardust THE CHEMISTRY OF Natural Products 1. Dont be concerned with which atom gave what.
Complete pairing of these e- is impossible and an octet around each atom cannot be achieved resonance structures 2. Generally electrons are paired. A polyatomic anion or cation add.
If the entire molecule is charged ie. 23 hours agoDraw the Lewis structures for three compounds with a formula of C3H8O. Only the outermost electrons are available for.
How to Draw a Lewis Structure. Multiple bonds are often formed with the following atoms. Write the skeletal structure.
Place Electrons Around Outside. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots. Subtract electrons for positively charged ions.
The group number of an element gives the number of electrons available for bonding valence number of electrons. Choose a Central Atom. Rules for Drawing Lewis Structures Complete the Lewis structures in the table on the datasheet.
If covalent treat the entire molecule. Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure. 1 Find the total number of valence electrons for a neutral molecule by adding up the number of valence electrons needed for each atom in the molecule.
Start studying Rules for drawing Lewis structures. Compounds of low electronegativity metals with high electronegativity nonmetals ΔEN 16 are ionic as are compounds of metals with polyatomic anions. If the charge is negative add electrons and if the charge is positive subtract electrons Step 2.
Determine whether the compound is covalent or ionic. Occurs when there are fewer than eight valence electrons around an atom resonance structures 3.

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

Lewis Structure Organic Chemistry Video Clutch Prep
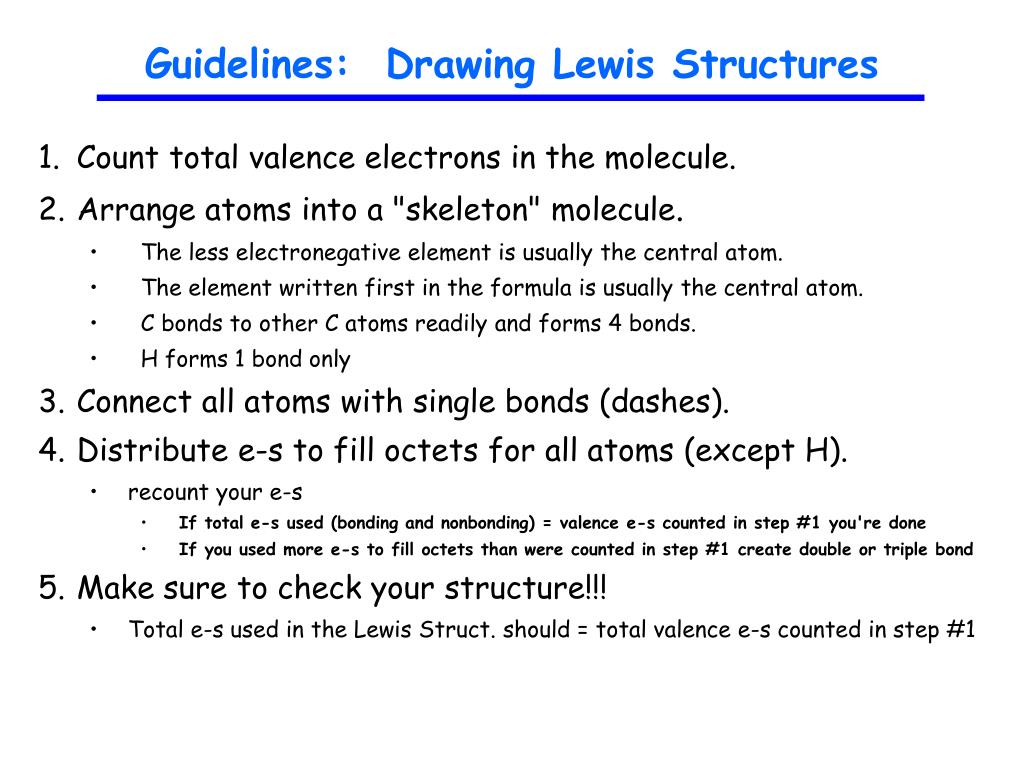
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Electron Dot Model Of Bonding Lewis Structures

I Can 2 I Can Draw A Lewis Structure Rules For Lewis Structures 1 Total Number Of Valance Electrons 2 Central Atom Always C Never H Rarely O Or Ppt Download


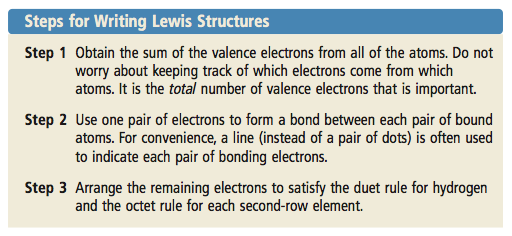
0 comments
Post a Comment